Table of Contents
The purposes of annealing are
(1) To soften steel so it can be machined more easily
(2) To relieve internal stresses that may have been caused by metalworking or uneven shrinkage in the casting.
The process involves
(A) Heat slowly to the required temperature
(B) Holding at this temperature long enough to allow internal changes to take place
(C) Cooling slowly. We have seen that above the critical range steel consists of austenite.
This is true whatever the structural condition of the steel before heating. Furthermore, when austenite is cooled normally in the critical range, it changes to pearlite, mixed with ferrite or cementite, depending on the carbon content of the steel.
This change occurs only if the cooling is slow. The true transformation to pearlite, therefore, depends on having true austenite, to begin with, and then allowing sufficient time for the metal to cool through the critical range for soft pearlite formation.
The temperature for annealing should be 30° to 50° above the highest critical point for steels up to 0•93 carbon, and approximately the same amount above the lower critical point for high carbon and tool steel* (above 0•93 C ). The reader can get them from the graph (Fig. 24), and they are approximated as follows:

The time it takes for the metal to reach oven temperature, and the length of time it must soak at the annealing temperature, varies depending on the shape and dimensions of the article, but the process should not be rushed.
The best method of cooling is to turn off the oven and leave the work inside, allowing the whole to cool slowly together. If this is not possible, the metal must be removed and buried in a non-conductive material such as sand, lime or ash.
Normalizing
The purpose of normalization is to refine the steel structure and remove stains that may have been caused by cold working. When steel is cold worked (hammered, rolled, bent, etc., in the cold condition) the crystal structure is distorted and the metal can be brittle and unreliable.
Furthermore, when steel is kept heated for a considerable period well above the highest critical point (as may often be required for prolonged forging), an increase in grain size occurs, and when cooled, the metal may have lost its tenacity. If the steel is slowly heated to its annealing temperature, the structure is in the most refined state and normalization consists of air-cooling it from this point.
Hardening and tempering of carbon steels
We now know that the critical range is the interval between two temperatures, the lowest being around 700°C, and that when steel cools normally in this range, it changes from austenite to pearlite, plus a free constituent. If we can somehow lower the temperature of the transformation of austenite so that instead of occurring at 700°, it occurs at about 300°, it doesn’t break down into pearlite but turns into a constituent called martensite.
We can lower this transformation temperature by cooling the metal suddenly, so that the change does not have time to take place at the normal point, but is forced to take place at some lower temperature.
Quenching is usually done by quenching the steel in water or some other liquid, and the efficiency with which quenching occurs determines how much of the austenite is transformed into martensite and the hardness of the steel.
The exact constitution of martensite is not clearly known, but it serves our purpose to know that it is a very hard substance capable of withstanding extreme wear and tear and cutting other metals. It has a needle-like structure as shown in Figure It should be clear that martensite cannot be formed (and steel hardened) by quenching until the steel is in the austenitic condition, i.e. above the lowest critical temperature, carbon then being in solution.
For this reason, the term hardening carbon was given to the form of carbon when steel is above the lowest critical temperature and carbon from cement when below this point (i.e. in pearlite). When the carbon is in the form of cement (softening), the steel cannot be hardened by quenching. Before moving on to the practical consideration of hardening and tempering, it will be necessary to qualify our observations for low carbon steels a little.
When steel contains less than 0.3% carbon, and this includes all mild steel, the solution of carbon in the iron that forms austenite is naturally a much weaker solution and contains more iron than high-carbon steel.
When steel of this grade is quenched, some of this extra iron is released into the structure and this, together with the fact that fewer carbon results in less martensite, makes it impossible for the mild steel to harden in the way just described. Mild steel will certainly be a little harder and more uniform in texture as a result of tempering, but it will not be hard in the generally accepted meaning.
Tempering
Martensite, being an extremely hard and brittle substance, makes a hard, dead tool made of it liable to cracks and splinters. The steel is therefore reheated to a temperature below the lowest critical temperature, which causes a partial transformation of martensite back into pearlite, thus removing some of the hardness but making the steel stronger.
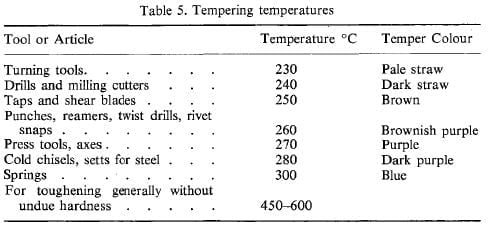
The temperature at which tempering should be carried out depends on the purpose for which the article or tool will be used, and the table below provides temperatures for some of the common high carbon steel applications.
When the article has been brought to tempering temperature, it can be cooled or cooled naturally. The temperature for this operation is often judged by the colour of the oxide film that appears on a freshly polished surface of the article, and these colours are given in
Quenching
We have seen that to efficiently transform austenite into martensite, the cooling must be so rapid that the transformation temperature is reduced from about 750°C to 300°C. It involves a lot of rapid cooling and brings troubles with distortion and cracking. There are two factors that tend to cause the metal to warp and crack:
(1) When the metal is cooled, it undergoes a general contraction that is not uniform, but occurs first on the outer surfaces and thin sections of the article.
(2) When the steel cools to the critical range, expansion occurs.
Now, if we could cool the metal so that its entire volume could be suddenly cooled at the same instant, we wouldn’t have too many problems with volume changes, but unfortunately, that’s not possible.
Let’s examine what happens: after carefully heating the steel to the annealing temperature, we quickly take it out of the oven and immerse it in water. The outer portion of the metal in contact with the water is immediately cooled and undergoes its critical range expansion, followed by its cooling contraction, becoming a hard, rigid metal skin.
The inner part of the metal, however, has not yet felt the effect of tempering and is still red-hot. A moment later, the extinction effect is transferred to this portion, which, when passing through the critical range, must expand. One can easily imagine what is likely to happen to the hard, brittle outer layer of cold metal when this inner core undergoes its critical expansion: it’s a matter of luck if it doesn’t crack, and most of us must have had that mortifying experience.
The general shrinkage that occurs can to some extent be allowed for by the direction in which we temper the tool. For example, if a long tool such as a faucet is tempered on its side, the entire length of one side meets the cold water at once and contracts. This sudden shortening of one side causes the faucet to deform, and we must remember that trying to straighten it afterwards will certainly break it.
If the faucet is dipped vertically in the water, the deformation effect is greatly minimized. The reader should study each work he has to harden and endeavour to erase it in such a way that the deformation effects are minimized as much as possible. When an article has been quenched, it should be moved through the water to accelerate cooling as much as possible.
After what has been said about the slower cooling of the inner metal of large and thick works, it can be imagined that the section of such an article will not have the same hardness throughout, and the centre itself might almost have had time to transform. for the pearlitic condition.
This may not be a disadvantage, as a softer core for a tool helps give it strength and we probably won’t require the use of metal near the centre for the cut. When extreme hardness can be sacrificed in favour of extra hardness, oil can be used for cooling instead of water, with less risk of cracking during quenching.
As the specific heat capacity of oil is less than that of water, its cooling effect is less and the quenching effect is not as intense. This means that less martensite will be formed and the tool will be softer and stronger.

























hey, outstanding blog on fatlike loss. aforesaid helped.
I have been exploring for a little for any high quality articles or blog posts in this kind of area . Exploring in Yahoo I ultimately stumbled upon this web site. Reading this information So i¡¦m glad to show that I have an incredibly good uncanny feeling I came upon exactly what I needed. I such a lot indubitably will make certain to don¡¦t forget this web site and give it a look a relentless basis.