Table of Contents
State joules Law of heating:
Joule’s law of heating states that the total heat energy generated in an electrical conductor is proportional to the square of the current passing through it and the resistance of the conductor. This law was first formulated in 1841 by James Prescott Joule and is also known as Joule’s Law of Heating.
It states that the heat generated, Q, in a conductor with a certain resistance, R, when a current, I, passes through it is given by the equation: Q = I2R. This law is based on the fact that electrical energy is converted into heat energy as it passes through a conductor.
It is a fundamental law of electrical circuits and is used to calculate the amount of heat produced in an electrical circuit. It is also used to calculate the power dissipated in a conductor when the current and resistance are known.
The state of Joule’s law of heating is an important law in electricity and is used to calculate the power dissipated in a conductor when the current and resistance are known. It is also used to calculate the amount of heat produced in an electrical circuit. This law is applicable to all types of conductors and is used in many electrical engineering applications.
The definition of joules Law:
Joule’s Law states that the amount of heat generated by an electrical current is proportional to the resistance of the conductor, as well as the square of the current and the time the current is flowing.
In other words, the amount of energy produced by a given current is directly proportional to the product of resistance, current, and time. This law was proposed by the English physicist James Prescott Joule in 1841 and is a fundamental law of electrical circuits.
Joule’s law is also known as the Joule-Lenz law, Joule-Thompson law, or Joule-Lenz-Thompson law. In mathematical terms, Joule’s law is expressed as Q=I2RT, where Q is the amount of energy produced in joules, I is the current flowing in amperes, R is the resistance of the conductor in ohms, and T is the time period the current is flowing in seconds.
This law is important for understanding the behavior of electrical circuits and for calculating the amount of heat generated by an electrical current. Joule’s law is also useful for determining the efficiency of electrical appliances, as it helps to determine how much energy is lost to heat when a current is flowing through a conductor.
The background of Joules Law:
Joule’s Law, also known as Joule-Lenz Law, was named after scientist James Prescott Joule. Joule was born in Salford, Lancashire, England. He was a scientific researcher who made significant contributions to the fields of thermodynamics and electricity.
Joule’s Law states that the work done by a system is equal to the amount of energy transferred to the system. This law was first proposed in 1843 when Joule conducted experiments in which he observed that the amount of heat produced by a current-carrying wire was proportional to the amount of electrical energy it was carrying.
This led him to conclude that heat and mechanical work were equivalent forms of energy. Joule’s Law is an important law in the study of thermodynamics and is used to calculate the amount of work done by a system. It is also used to calculate the efficiency of a system, as well as to analyze the effects of temperature on a system.
In addition, Joule’s Law is also used in the study of electricity, as it can be used to calculate the power of a circuit. Joule’s Law has since been used in many other scientific fields, including chemistry, engineering, and economics. It is a fundamental law that is used to understand the relationships between energy and work.
The state Joules Law of heating Theory:
Joule’s Law of Heating Theory states that the amount of heat produced by a current flowing through a conductor is equal to the product of the current squared, the resistance of the conductor, and the time for which the current is allowed to flow.
This law can be expressed mathematically as Q = I2Rt, where Q is the amount of heat produced, I is current, R is the resistance of the conductor, and t is the time. Joule’s Law of Heating Theory is based on the fact that when a current is passed through a conductor, it generates heat.
This heat is a result of the electrical energy being converted into thermal energy due to the resistance of the conductor. The amount of heat produced is directly proportional to the current squared and the resistance of the conductor.
As the current flows through the conductor, the resistance causes the current to dissipate, resulting in the conversion of electrical energy into thermal energy. Joule’s Law of Heating Theory has many practical applications.
For example, it is used to calculate the amount of heat produced by a current flowing through a resistor. It is also used to determine the amount of heat generated by an electrical appliance and to determine the efficiency of electrical heating systems.
Joule’s Law of Heating Theory is an important part of electrical engineering and is used in many applications. This law is a simple and effective way of understanding how electrical energy is converted into heat. It is important to understand this law in order to design efficient electrical systems and to calculate the amount of heat that can be produced by a given current.
The equation of Joule’s Law:
Joule’s Law states that the heat generated in a conductor is proportional to the square of the current passing through it. This law was first proposed by English physicist James Prescott Joule in 1841.
The equation for Joule’s Law is expressed as Q = I2Rt Where ‘Q’ represents the heat generated (in Joules), ‘I’ is the current (in Amps), ‘R’ is the resistance of the conductor (in Ohms) and ‘t’ is the time (in seconds) for which the current is applied.
In other words, the heat generated in a conductor is directly proportional to the square of the current passing through it and is also proportional to the resistance of the conductor and the duration for which the current is applied.
For example, if a current of 10 Amps is passed through a conductor of resistance 10 Ohms for a duration of 10 seconds, the heat generated can be calculated using the equation as Q = (10)2 (10) (10) = 10,000 Joules Therefore, the heat generated in this case will be 10,000 Joules. This can be verified by measuring the temperature rise of the conductor after the current is applied.
The Applications of Joule’s law:
Joule’s law states that the heat generated by a current flowing through a resistor is directly proportional to the square of the current and the resistance of the resistor.
This law is named after the British physicist Sir James Prescott Joule who discovered the law in 1841. This law forms the basic principle for the operation of electrical appliances such as electric heaters, electric motors, electric irons, and even incandescent light bulbs. Joule’s law has various applications in modern-day electrical gadgets.
For example, electric irons use Joule’s law to generate heat. When an electric current passes through the heating element of the electric iron, it gets heated up due to Joule’s law. Similarly, in electric motors, Joule’s law helps in the generation of mechanical energy.
As the electric current passes through the motor’s coil, it generates a magnetic field, due to which the coil rotates, producing mechanical energy. Joule’s law is also used in electric heating elements.
When a current passes through the element, it gets heated up, and this heat is used to heat up the surrounding. This is the principle used in electric water heaters, electric radiators, and electric cookers. Joule’s law is also used in the design of incandescent light bulbs.
When an electric current passes through the filament of the bulb, it gets heated up and emits light due to Joule’s law. Apart from these applications, Joule’s law can also be used to calculate the energy consumed by a device.
By measuring the current and the resistance of a device, one can calculate the amount of energy consumed by the device. This calculation is done by multiplying the current with the square of the current and then multiplying the result with the resistance. This is known as Joule’s law equation.
The Electrical heating in state joules law of heating:
Joule’s Law of Heating states that the heat produced by an electrical current is proportional to the square of the current and is proportional to the resistance of the conductor.
This means that if the current is doubled, the heat produced will be quadrupled and if the resistance is doubled, the heat produced will be quadrupled. Joule’s law of heating is also known as Joule’s law of electrical heating.
Joule’s law of heating is based on the principle of conservation of energy. It states that when electrical energy is converted into heat, the amount of heat produced is equal to the amount of electrical energy input.
This law is applicable to all electrical heating devices, including electric radiators, hot plates, electric ovens, and immersion heaters. Joule’s law of heating can be expressed mathematically as Q = I2Rt where Q is the amount of heat energy produced (in joules), I is the current (in amps), R is the resistance (in ohms) and t is the time (in seconds).
Joule’s law of heating is important for understanding the behavior of electrical heating devices. By applying it, engineers can determine the amount of heat produced and design efficient heating systems.
It is also used to calculate the amount of power required for a given heating system, taking into account the resistance of the conductor, the current, and the time.
The Joules heating in the state joules law of heating:
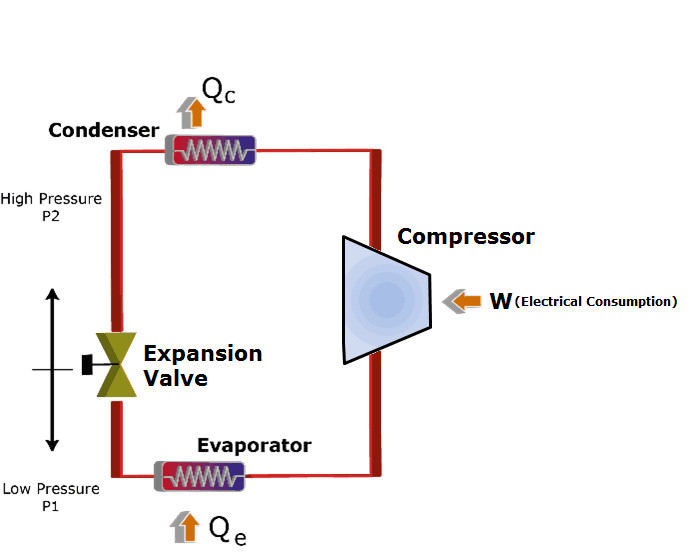
The Joule heating, also referred to as the Joule-Thomson effect, is a principle of thermodynamics that describes the heating of a gas as it passes through a throttling valve. It is named after James Joule, who discovered the effect in 1845.
The Joule-Thomson effect occurs when a gas is passed through a throttling valve, such as a regulator or a nozzle. As the gas passes through the valve, its pressure decreases, causing its temperature to drop.
This drop in temperature is known as the Joule-Thomson effect. When the temperature of the gas drops, its molecules tend to lose energy, which is then transferred to the surrounding molecules.
This transfer of energy results in a net increase in the temperature of the gas, which is known as Joule heating. The Joule-Thomson effect is a reversible process, meaning that the temperature of the gas can be increased or decreased depending on the direction of flow.
If the gas is flowing in the same direction as the throttling valve, then the temperature of the gas will decrease. Conversely, if the gas is flowing in the opposite direction of the throttling valve, then the temperature of the gas will increase.
This means that the Joule-Thomson effect can be used to either cool or heat a gas, depending on the direction of flow. The Joule-Thomson effect is an important principle of thermodynamics, as it is used in many industrial and scientific applications.
In particular, it is used in refrigeration and air conditioning systems, where it is used to cool air or other gases. In addition, it is also used in cryogenics and cryosurgery, where it is used to cool materials down to very low temperatures.
Finally, it is also used in gas turbines, where it is used to preheat the fuel before it is burned.
The Advantages of state joules law of heating:
Joule’s Law of Heating states that the rate of heat energy produced in an electrical circuit is equal to the electrical current multiplied by the resistance of the circuit. This law is widely used to calculate the amount of heat produced by an electrical device or system. There are several advantages to Joule’s Law of Heating.
Firstly, it is a simple and reliable way of determining the exact amount of energy produced by an electrical device or system. This makes it easier to accurately assess the efficiency of a device or system and to make decisions about how much energy should be used to power it.
Secondly, Joule’s Law of Heating can help to prevent the overloading of electrical circuits, which can lead to dangerous electrical fires. By understanding the exact amount of energy produced in a circuit, it is easier to ensure that the circuit is not overloaded.
Thirdly, Joule’s Law of Heating can be used to accurately predict the amount of heat that will be produced by an electrical device or system. This means that designers can accurately design their products with the right amount of heat output, which can help to improve their efficiency and reduce their costs.
Finally, Joule’s Law of Heating can be used to calculate the amount of energy that is required to heat a particular space. This is particularly useful for heating systems, as it can help to ensure that the correct amount of energy is used to heat a room or building.
Overall, Joule’s Law of Heating is a simple and reliable way of measuring the amount of energy produced by an electrical device or system. It provides a simple way of calculating the amount of energy required to heat a specific space and can help to prevent electrical fires.
It can also be used to accurately predict the amount of heat that will be produced by a device or system, making it easier to design efficient and cost-effective products.
The disadvantages of state joules law of heating:
State Joule’s law of heating states that the rate of heat flows through a given material is proportional to the product of its cross-sectional area and the square of the difference in temperature between its two surfaces. While this law is useful for understanding heat transfer in many situations, it does have some disadvantages.
First, State Joule’s law does not take into account the effect of various thermal resistances such as convection, conduction, and radiation. These are important factors in the heat transfer process and can significantly affect the amount of heat that is transferred.
Second, State Joule’s law does not account for the effect of certain materials on the rate of heat flow, such as insulation and air pockets. These materials can significantly reduce the rate of heat transfer and should be taken into consideration when designing heat transfer systems.
Third, State Joule’s law assumes that the material is homogenous and that the heat transfer process is linear. This is not always the case and can lead to inaccurate predictions of heat transfer.
Finally, State Joule’s law does not take into account the effect of a material’s thermal conductivity on the rate of heat transfer. The thermal conductivity of a material can have a significant impact on the rate of heat transfer and should be taken into consideration when designing heat transfer systems.
Overall, State Joule’s law of heating can be a useful tool for understanding heat transfer, but it should not be relied upon exclusively. It is important to take into account other factors such as thermal resistances and thermal conductivity, when designing heat transfer systems.
The conclusion of the state joules law of heating:
State Joule’s law of heating states that the rate of heat that flows through a material is directly proportional to the product of the thermal conductivity of the material and the temperature gradient across the material.
This law states that the amount of heat transferred per unit of time is proportional to the difference in temperature between two points and the thermal conductivity of the material between two points.
The conclusion of State Joule’s law of heating is that the rate of heat flow is directly proportional to the thermal conductivity of the material and the temperature difference between two points. This means that if the thermal conductivity of the material increases, the rate of heat flow will also increase.
Similarly, if the temperature difference between two points increases, the rate of heat flow will also increase. This law has been found to be valid for most materials, with the exception of some special cases.
In conclusion, State Joule’s law of heating can be used to determine the rate of heat flow through a material.
The References of state joules law of heating:
The Joule–Thomson effect, also known as the Joule–Kelvin effect, is a thermodynamic phenomenon in which there is a temperature change in a gas when it is allowed to expand freely.
This effect was discovered by James Prescott Joule and William Thomson (Lord Kelvin) in 1852. The Joule–Thomson effect is based on the Joule–Laws of Thermodynamics which states that energy is conserved during a thermodynamic process.
It is useful in determining the temperature changes in a gas when it is allowed to expand from a high-pressure region to a low-pressure region. The Joule–Thomson coefficient is a measure of the rate of temperature change and is determined experimentally.
The Joule–Thomson effect is important for several reasons. It is used in the design of gas turbines and other power plants to ensure efficient operation. It is also important for controlling the temperature of gases in chemical processes.
The Joule–Thomson effect can be used to measure the enthalpy of a gas. This is done by measuring the temperature change of a gas as it is allowed to expand from a high-pressure region to a low-pressure region. The enthalpy can then be calculated from the measured temperature change and the known Joule–Thomson coefficient.
The Joule–Thomson effect is also used to determine the thermodynamic properties of a gas. When a gas is allowed to expand, its temperature, pressure, and volume all change. By measuring these changes, the thermodynamic properties of the gas can be determined.
The Joule–Thomson effect is an important principle in the study of thermodynamics and is useful in many different applications. It is used in the design of power plants, chemical processes, and determining the thermodynamic properties of gases.