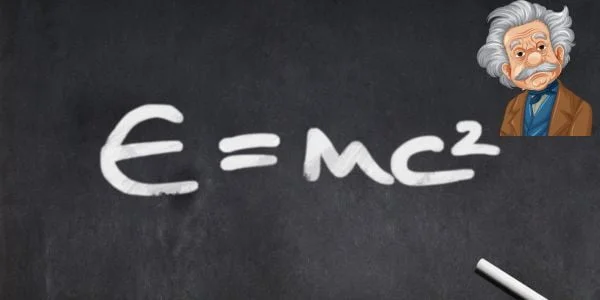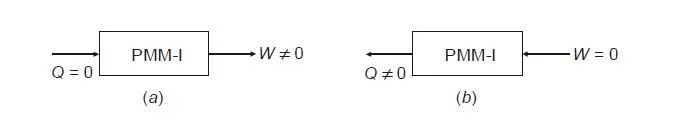Table of Contents
Law of Conservation of Energy:
The Law of Conservation of Energy states that “Energy cannot be created nor destroyed in an isolated system; it can only be transformed from one form to another or transferred between objects”. This principle is critical for understanding numerous natural phenomena, providing the foundation for various scientific theories and technological advancements.
The seeds of the conservation of energy principle were sown in the 19th century with the studies of heat and work. As researchers like Julius Robert von Mayer, James Joule, and Hermann von Helmholtz delved deeper into understanding the nature of energy, it became increasingly clear that energy, in its various forms, remains consistent throughout transformations.
Different Forms of Energy:
To better understand the law, it’s essential to recognize the various forms of energy:
- Kinetic Energy: The energy of motion. It’s calculated using the formula: KE=1/2mv2, where m is mass and v is velocity.
- Potential Energy: Energy stored due to position or configuration, often seen in gravitational or elastic situations.
- Thermal Energy: The total internal energy of an object due to the kinetic motion of its atoms and molecules.
- Chemical Energy: Energy stored within the bonds of chemical compounds.
- Nuclear Energy: Energy stored within the nucleus of an atom, released during nuclear reactions.
- Electromagnetic Energy: Energy associated with electric and magnetic fields.
These are just some of the primary forms; energy can manifest in numerous other ways.
Mathematical Representation: For any closed system, the total energy E is constant. If the system undergoes a transformation, the change in its internal energy ΔU is equal to the heat Q added to the system minus the work W done by the system on its surroundings:
ΔU=Q−W

Applications and Examples:
- Rolling Ball: Consider a ball placed at the top of a hill. Initially, it has high potential energy and zero kinetic energy. As it rolls down, the potential energy decreases while the kinetic energy increases, but the total energy remains constant.
- Pendulum Motion: A swinging pendulum at its highest point has maximum potential energy and zero kinetic energy. At its lowest point, it has maximum kinetic energy and minimal potential energy.
- Chemical Reactions: In a chemical reaction, the energy stored in the reactant molecules is transferred to the product molecules and the surroundings. No energy is lost or gained; it’s merely redistributed.
- Nuclear Reactions: In nuclear fusion or fission, the energy produced isn’t from the creation of new energy but the conversion of mass into energy, as represented by Einstein’s famous equation E=mc2.

Implications and Consequences:
- Perpetual Motion Machines: The conservation of energy law negates the possibility of creating a perpetual motion machine of the first kind, which would create its own energy, thereby violating this principle.
- Energy Management: On a global scale, even though energy is conserved, the forms in which we use energy can render it less useful. For example, burning fossil fuels converts chemical energy into thermal energy, which then dissipates into the surroundings, becoming challenging to harness again.
Challenges and Limitations:
It’s crucial to note that the conservation law applies to isolated systems. In the real world, there are very few truly isolated systems, which means energy can seem to “disappear” in certain scenarios. However, this is usually due to the energy becoming too dispersed or being transformed into a form that’s challenging to detect.
The Law of Conservation of Energy is paramount in the realm of physics, offering insights into the behavior of everything from tiny atoms to vast galaxies. By understanding that energy remains constant but can change forms, we can better comprehend and predict the behavior of systems and phenomena around us. Whether considering the simple fall of an apple or the complexities of a star’s life cycle, the conservation of energy principle provides a guiding light for our understanding.
Also, read Diary of a Wimpy Kid