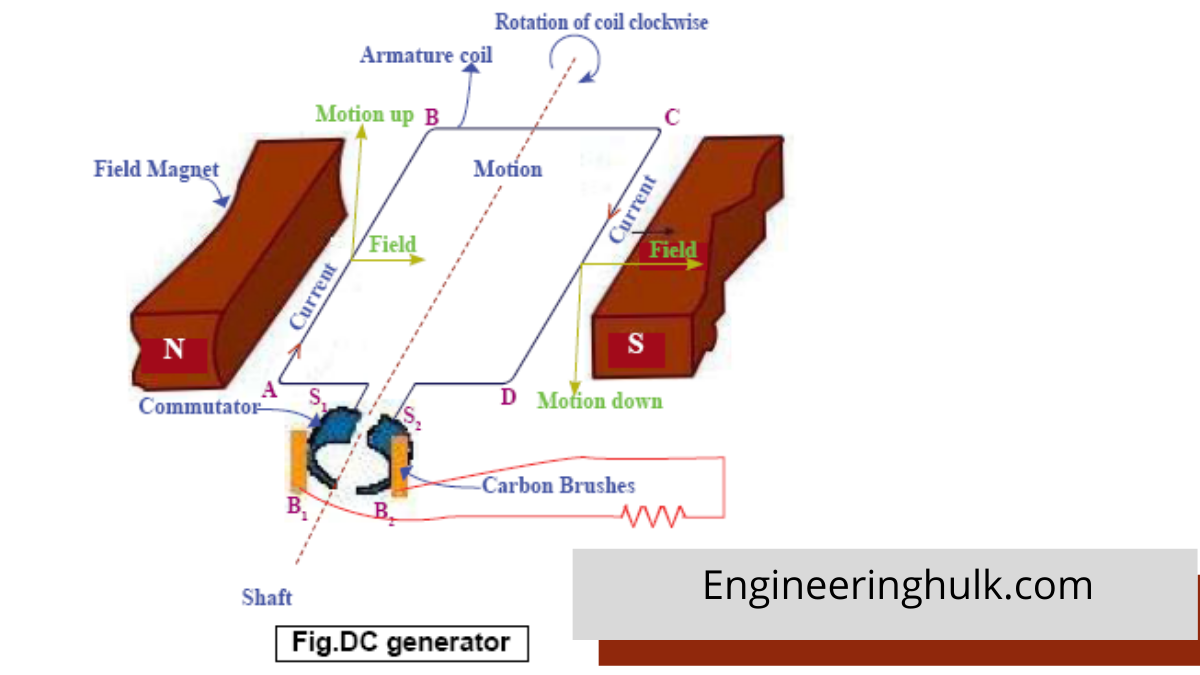
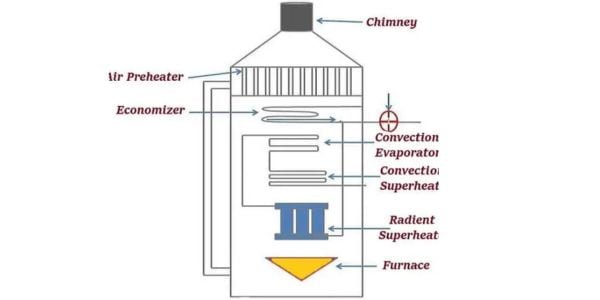
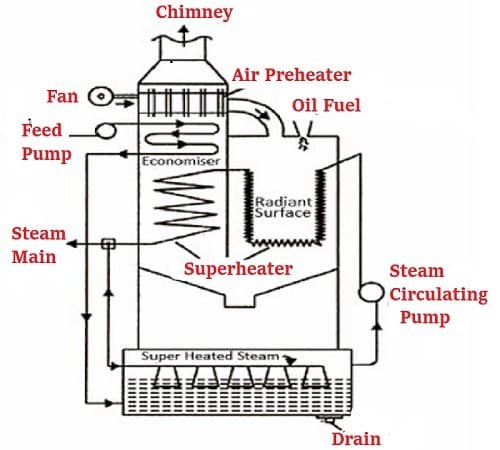
Table of Contents
LIMITATIONS OF THE FIRST LAW OF THERMODYNAMICS
The first law of thermodynamics based on the law of conservation of energy has proven to be a powerful tool for thermodynamic analysis. But over the time that it has been applied to some real systems, it has been observed that, theoretically, the first law holds for processes that are not feasible in practice. So it was thought that there were certain flaws in the first law of thermodynamics and that it should be used with certain limitations.
Say, for example, let’s take a bicycle wheel and paddle to turn it. Now apply the brake on it. As a result, the brake wheel stops when it comes into contact with the brake shoe. The wheel stop is accompanied by the heating of the brake shoe. Examining the situation from the point of view of the first law of thermodynamics, it is quite satisfactory that the rotational energy in the wheel has been transformed into thermal energy with the shoe, which causes its temperature to increase:
Now, if we want to put the same amount of heat into the brake shoe and we want to restore wheel motion, it is simply not possible, whereas theoretically, the first law allows for the conversion of heat into work (the rotation of the wheel in this case) as well.
Therefore, it is obvious that the first law of thermodynamics has certain limitations, as indicated below:
(i) The first law of thermodynamics does not differentiate between heat and work and guarantees full convertibility of one into the other, whereas full conversion of work to heat is possible, but vice versa is not possible.
(ii) The first law of thermodynamics does not explain the direction of a process. As theoretically, it should allow uniform heat transfer from a low-temperature body to a high-temperature body, which is not feasible in practice. The spontaneity of the process does not comply with the first law of thermodynamics.
A perpetual motion machine of the first type (PMM-I) is a hypothetical device conceived, based on a violation of the first law of thermodynamics. Let us think of a system that can create energy as shown below.
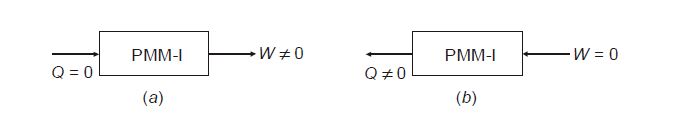
Here at
(a) a device is shown that continuously produces work without any other form of energy being supplied to it, which is not feasible.
Similarly, in
(b) a device is shown that continuously emits heat without any other form of energy being supplied to it, which again is not feasible.
The two imaginary machines above are called perpetual motion machines of the first kind.