Table of Contents
Schottky Defects are an important concept of physical chemistry. They can exist in various materials, from solids to metals and insulators to plasmas.
This comprehensive guide will explain the basics of Schottky Defects, how they manifest in different environments, and why they are important for understanding the nature of matter.
Further discussion will be devoted to how Schottky Defects appear in solids, gases, liquids, plasmas, metals, semiconductors, insulators, and superconductors.
Finally, a summary highlighting key points about various kinds of Schottky Defects will be provided at the end for reference purposes.
What is a Schottky Defect?
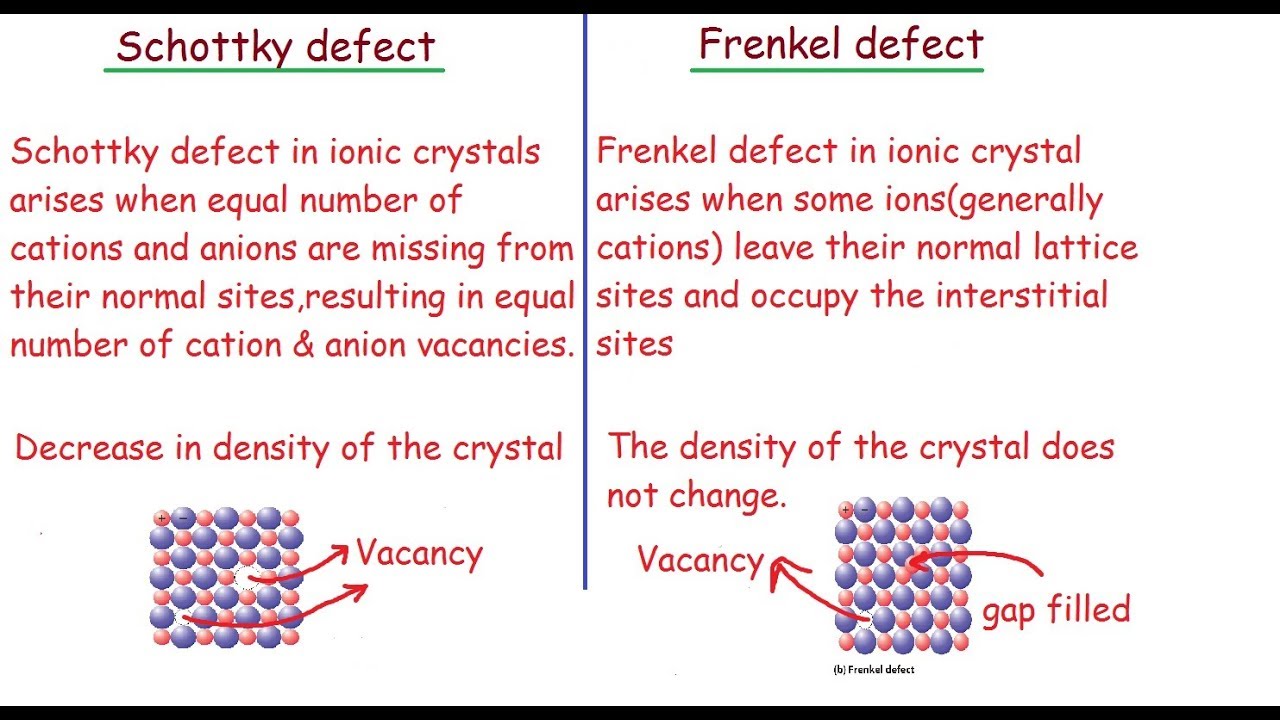
A Schottky Defect is an imperfection in a material’s structure caused by a mismatch between the number of cations and anions.
This imbalance can occur due to either excess or deficient numbers of the two ions, resulting in a net charge imbalance within the material.
In order for this type of defect to exist, there must be an atomic vacancy created which then allows electrons to move freely through the lattice structure.
These free-moving electrons are known as “Schottky Fermions” and are responsible for many electrical properties exhibited by materials with Schottky defects such as increased conductivity and decreased band gaps.
It has been suggested that these defects may play important roles in catalyzing processes such as oxidation reactions, making them highly important for many applications.
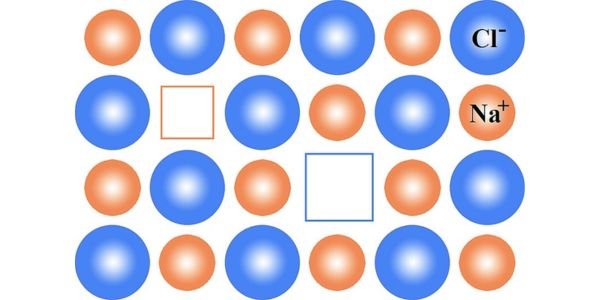
Schottky Defects in Solids
Schottky Defects in solids are particularly important for understanding the structure and behavior of materials. The most common type of Schottky defect is a vacancy-type defect, where there is an unequal distribution of cations and anions on lattice sites, resulting in a net charge imbalance.
In this case, electrons can move freely through the lattice structure as “Schottky Fermions, ” allowing them to exhibit increased conductivity and decreased band gaps. This makes Schottky defects desirable for certain applications, such as catalyzing oxidation reactions or creating high electron mobility transistors (HEMTs).
Furthermore, these types of defects have been observed in various material systems, including metals and semiconductors, making them highly relevant to many industries.
In addition to their importance from an industrial standpoint, Schottky Defects also provide insight into the physical properties that govern materials at nanoscale levels.
By studying how these defects form and interact with other atoms within a material’s lattice structure, researchers are able to better understand its macroscopic characteristics, such as electrical conduction or optical absorption behavior.
Consequently, understanding how different types of Schottky Defects manifest in various materials has become essential for designing new technologies with tailored properties that meet desired specifications.
Schottky Defects in Gases
Schottky Defects in gases can be observed in a wide range of materials, from rarefied monatomic gases to dense molecular mixtures. In these systems, most defects are created by an odd number of electrons or ions present within the system.
These imbalanced molecules become charged and interact with other particles resulting in ionization and electron transport which can modify the gas’s electrical properties, such as its conductivity or dielectric constant.
Additionally, Schottky Defects are known to induce chemical reactions when interacting with certain molecules.
This is due to their ability to act as catalysts for many processes, like oxidation-reduction reactions that are not normally possible under normal conditions.
Therefore, understanding how Schottky Defects manifest in different gaseous environments is important for designing new technologies that rely on their unique characteristics for specific uses, such as catalyzing reactions.
The presence of Schottky Defects can also lead to interesting physical phenomena occurring at low temperatures called Bose-Einstein condensation (BEC). Here, a large fraction of particles occupies the same quantum state leading them all into one single macroscopic wave function instead of being distributed among multiple states.
Understanding this phenomenon has allowed researchers to explore applications ranging from superconductors and superfluids through atom lasers and atomic clocks – all made possible by studying Schottky defects in gases!
Schottky Defects in Liquids
Schottky defects in liquids can be observed when there is an imbalance of positive and negative charges within the liquid, usually due to a decrease in solubility or an increase in temperature.
These imbalances create regions of electric potential, which tend to attract ions from the surrounding environment and form clusters known as Schottky Defects.
Such defects are important for understanding how different materials behave at nanoscale levels since their presence modifies electrical properties such as conductivity, capacitance, and dielectric constant.
Additionally, these defects have been observed in various systems, including ionic liquids, electrolyte solutions, and polymer melts, making them highly relevant for many industries.
In addition to being useful for practical applications like controlling electric fields or forming superconductors with low critical temperatures, Schottky Defects can also provide insight into the physical properties that govern materials on small scales.
For example, by studying how certain molecules interact with these defect clusters, it may be possible to gain valuable insight into their structure-property relationships, which could then be used to design new materials tailored specifically toward desired specifications.
Schottky Defects in Plasmas
Schottky Defects in plasmas are small regions of imbalanced positive and negative charges caused by an odd number of electrons or ions. These defects can modify plasma’s electrical properties, such as its conductivity, dielectric constant, and capacitance.
Additionally, due to their ability to act as catalysts for certain processes like oxidation-reduction reactions, understanding how these types of defects manifest in different environments has become essential for designing new technologies with tailored properties that meet desired specifications.
Plasma Schottky Defects have been studied extensively in various contexts ranging from astrophysics to nanotechnology.
In astronomy, they provide insight into the formation and evolution of stars while on the nanoscale, they help researchers better understand the behavior of materials at very small scales where traditional methods may no longer be applicable.
Such research is highly relevant for many industries since it allows us to design new devices with unique characteristics – such as atom lasers or optical devices with precise control over light transmission through liquid media – which could not be achieved without exploiting Schottky Defects within plasmas.
Consequently, further investigation into this phenomenon promises valuable knowledge that will lead to more efficient products and services going forward.
Schottky Defects in Metals
Schottky Defects in metals are regions of imbalanced positive and negative charges which manifest due to their relatively high temperature or an increase in solubility.
These defects can modify the electrical properties of the metal, such as conductivity, capacitance, and dielectric constant. Furthermore, they are crucial in many technologies ranging from optoelectronics to superconductors.
Due to their importance for various commercial applications, Schottky Defects within metals have been studied extensively over the past few decades providing us with valuable insight into how these types of defects interact with different materials at nanoscale levels.
Such understanding has enabled researchers to explore novel designs based on exploiting their unique characteristics – like creating atom lasers or optical devices with precise control over light transmission through liquid media – all made possible by studying Schottky Defects present within metallic environments.
Schottky Defects in Semiconductors
Schottky Defects in semiconductors are small regions of imbalanced positive and negative charges caused by the presence of an odd number of electrons or ions.
These defects can modify a semiconductor’s electrical properties, such as its conductivity, dielectric constant, and capacitance.
Research into how these types of defects manifest within various materials has become essential for designing technologies that exploit their unique characteristics – such as creating atom lasers or optical devices with precise control over light transmission through liquid media.
Schottky Defects in superconductors
Schottky Defects in superconductors are small regions of imbalanced positive and negative charges caused by an odd number of electrons or ions. These defects can significantly modify a material’s electrical properties, such as its conductivity, dielectric constant, and capacitance.
Schottky defects are a type of energy state, or point defect, present in materials and can occur in almost every type of material. They give vital information about how a system behaves and can be useful in determining material properties.
The idea that each specific material has different types of defects can give great insight into how to improve the quality or performance of materials used in many modern applications.
FAQ’S
What are Schottky defects?
Schottky defects are point defects in a crystalline structure where pairs of oppositely charged ions are missing from their lattice positions.
How do Schottky defects form?
Schottky defects form in ionic compounds when pairs of cations and anions are simultaneously missing from their lattice sites, resulting in a neutral charge in the crystal.
What are the effects of Schottky defects on the properties of materials?
Schottky defects reduce the density of the material and affect its electrical conductivity. They also affect the mechanical and optical properties, as they can influence the material’s crystal structure and lattice vibrations.
Can Schottky defects occur in non-ionic compounds?
No, Schottky defects are specific to ionic compounds where there is a significant difference in electronegativity between the cations and anions.
What factors can influence the formation of Schottky defects?
Temperature, pressure, and the presence of impurities or dopants can influence the formation of Schottky defects. Higher temperatures and the presence of impurities can promote defect formation.
Can Schottky defects be intentionally created or controlled?
Yes, Schottky defects can be intentionally created or controlled through various methods, such as doping with foreign atoms or adjusting the processing conditions during material synthesis.
Are Schottky defects reversible?
Yes, Schottky defects are generally reversible. When the appropriate conditions are provided, such as annealing at high temperatures, the missing ions can migrate back to their original lattice positions, resulting in the elimination of the defects.
References
- Ashcroft, N. W., & Mermin, N. D. (1976). Solid State Physics. Brooks/Cole.
- Chapter 24: Point Defects in Crystals
- Kittel, C. (1996). Introduction to Solid State Physics. Wiley.
- Chapter 7: Defects in Crystals
- Rao, C. N. R., & Gopalakrishnan, J. (2004). Concepts of Physics. New Age International.
- Chapter 16: Crystal Imperfections
- O’Keeffe, M., & Hyde, B. G. (1985). Defects and Geometry in Condensed Matter Physics. Academic Press.
- Chapter 3: Point Defects
- Stoneham, A. M. (1975). Theory of Defects in Solids: Electronic Structure of Defects in Insulators and Semiconductors. Oxford University Press.
- Chapter 5: Point Defects in Insulators
Also, read d el ed full form