Table of Contents
The definition of VSEPR theory:
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms.
It is also known as the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The basic premise of the VSEPR Theory is that the valence electron pairs surrounding an atom will repel each other, causing them to be as far apart as possible.
This repulsion causes the electrons to adopt a certain molecular geometry that can be predicted based on the number of electron pairs around the central atom. The VSEPR Theory is used to predict the geometry of a molecule by determining the number of electron pairs around its central atom.
It assumes that all the electron pairs around the central atom are repulsing each other, causing them to spread out as far as possible. This repulsion determines the shape of the molecule, which is typically described in terms of the number of bonds and lone pairs around the central atom.
For example, a molecule with two electron pairs around the central atom will have a linear shape, while one with three electron pairs will have a trigonal planar shape.
The VSEPR Theory can also be used to predict the shapes of more complicated molecules, such as those with multiple central atoms. Overall, the VSEPR Theory is a useful tool for predicting the shapes of molecules, and it is widely used in chemistry.
The history of VSEPR theory:
The VSEPR (Valence Shell Electron Pair Repulsion) theory was first proposed in 1957 by the British chemists Ronald Gillespie and Neil N. Greenwood. The concept of VSEPR was developed to explain the shapes of molecules and ions by assigning electron pairs around a central atom.
By predicting the shapes of molecules, chemists can more accurately predict the physical and chemical properties of molecules. The VSEPR model is based on the idea that the valence shell electron pairs around a central atom will repel each other to minimize the energy of the system.
The electron pairs will move as far away from each other as possible. The more electron pairs around the central atom, the more angles they must be placed in to minimize repulsion between them. This will determine the shape of the molecule.
For example, the water molecule, H2O, has four electron pairs around the central oxygen atom. The four electron pairs form a tetrahedral shape with a bond angle of 109.5°. This is because the four electron pairs repel each other as far away from each other as possible, which creates the tetrahedral shape.
The VSEPR theory is not perfect and there are many exceptions, such as when molecules have lone pairs of electrons or resonance structures. In addition, the theory does not take into account the effects of bonding orbitals or molecular orbitals.
However, the VSEPR theory is still widely used as a simple and effective way to predict molecular shapes and properties.
What is VSEPR theory?
The VSEPR theory (Valence Shell Electron Pair Repulsion) is a model used in chemistry to explain the shapes of molecules or ions. It is based on the idea that the valence electron pairs surrounding an atom will repel each other, and the molecule or ion will assume a shape that minimizes this repulsion.
The VSEPR theory states that the arrangement of atoms in a molecule can be predicted by considering the number of electron pairs in the valence shell of the central atom in the molecule. The number of electron pairs is the same as the number of bonds that the atom forms.
According to the VSEPR theory, the electron pairs will spread out as far as possible to minimize the repulsion between them. The shapes of molecules can be determined by considering the number of electron pairs around the central atom and the type of bonding that occurs.
If the molecule has two electron pairs, it will have a linear shape. If it has three electron pairs, the shape will be trigonal planar. If it has four electron pairs, the shape will be tetrahedral. If it has five electron pairs, the shape will be trigonal bipyramidal. And if it has six electron pairs, the shape will be octahedral.
The VSEPR theory is a useful tool for predicting the shapes of molecules or ions. However, it is important to note that the VSEPR theory does not take into account the presence of lone pairs on the central atom, which can affect the shape of the molecule.
In addition, the VSEPR theory is only applicable to molecules or ions in which all the atoms have the same electronegativity; if the electronegativity of the atoms is different, the shapes of the molecules will be distorted.
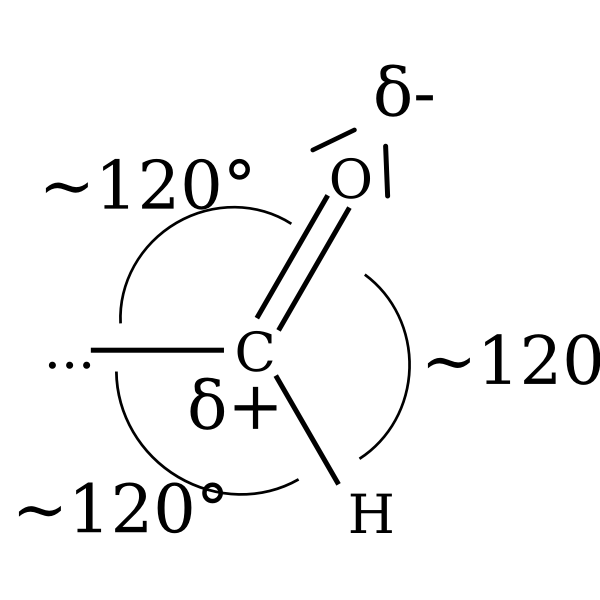
The molecular geometry in VSEPR theory:
The valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the shape of individual molecules based on the repulsion between the electron pairs surrounding the central atom.
According to this theory, the geometry of a molecule is determined by the number of electron pairs surrounding the central atom. These electron pairs can be either lone pairs (which are non-bonding electron pairs) or bonding pairs (which are shared with another atom).
The number of electron pairs determines the basic geometry of the molecule. If there are two electron pairs, the molecule is linear. If there are three electron pairs, the molecule is trigonal planar. If there are four electron pairs, the molecule is tetrahedral.
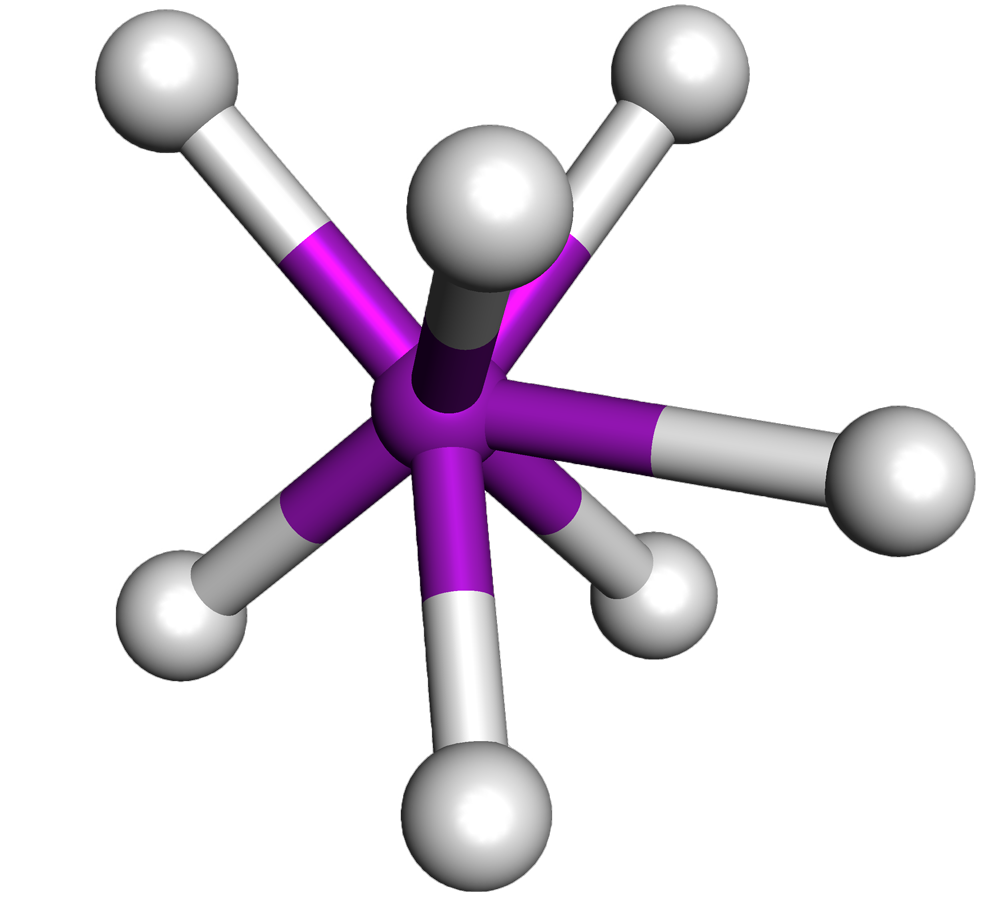
If there are five electron pairs, the molecule is trigonal bipyramidal. If there are six electron pairs, the molecule is octahedral. The VSEPR theory also takes into account the repulsion between the electron pairs. When two electron pairs are present, they will repel each other and will be as far apart as possible.
This will lead to a linear geometry, with the two electron pairs at 180° from each other. When three electron pairs are present, they will repel each other and be as far apart as possible. This will lead to a trigonal planar geometry, with the three electron pairs at 120° from each other.
When four electron pairs are present, they will repel each other and be as far apart as possible. This will lead to a tetrahedral geometry, with the four electron pairs at 109.5° from each other. When five electron pairs are present, they will repel each other and be as far apart as possible.
This will lead to a trigonal bipyramidal geometry, with the five electron pairs at 90°, 120°, and 180° from each other. When six electron pairs are present, they will repel each other and be as far apart as possible. This will lead to an octahedral geometry, with the six electron pairs at 90° from each other.
The VSEPR theory can be used to predict the shape of a molecule based on the number and type of electron pairs surrounding the central atom. The repulsion between the electron pairs will determine the geometry of the molecule.
The VSEPR theory is a useful tool for understanding the structure of molecules and can be used to predict the shape and arrangement of atoms in a molecule.
Examples of VSEPR theory:
The VSEPR (Valence Shell Electron Pair Repulsion) theory is a model used to predict the molecular geometry of a molecule based on the repulsion of the electron pairs surrounding the central atom.
This theory was proposed by Sidgwick and Powell in 1940 and was later improved by Gillespie and Nyholm in 1957. The VSEPR theory is based on the assumption that the electron pairs surrounding an atom will repel each other, and, as a result, the geometry of the molecule will be determined by the relative positions of these electron pairs.
One example of the VSEPR theory is the water molecule (H2O). According to the VSEPR theory, the two hydrogen atoms in the water molecule will be located on the two opposite sides of the oxygen atom.
This is because the two hydrogen atoms are the only electron pairs surrounding the oxygen atom, and they will repel each other and move to opposite sides of the oxygen atom in order to minimize the repulsion.
As a result, the water molecule will have a bent molecular geometry, with the oxygen atom in the center and the two hydrogen atoms located at the two opposite sides of the oxygen atom.
Another example of the VSEPR theory is the ammonia molecule (NH3). According to the VSEPR theory, the three hydrogen atoms in the ammonia molecule will be located at the three vertices of a trigonal pyramid, with the nitrogen atom at the center.
This is because the three hydrogen atoms are the only electron pairs surrounding the nitrogen atom, and they will repel each other and move to the three vertices of a trigonal pyramid in order to minimize the repulsion.
As a result, the ammonia molecule will have a trigonal pyramidal molecular geometry, with the nitrogen atom at the center and the three hydrogen atoms located at the three vertices of the trigonal pyramid. In conclusion, the VSEPR theory is a model used to predict the molecular geometry of a molecule based on the repulsion of the electron pairs surrounding the central atom.
Examples of the VSEPR theory include the water molecule (H2O) and the ammonia molecule (NH3). In both cases, the electron pairs surrounding the central atom will repel each other and move to positions that minimize the repulsion, resulting in the expected molecular geometries.
The Applications of VSEPR theory:
VSEPR (Valence Shell Electron Pair Repulsion) theory is a model in chemistry that is used to predict the shapes of molecules. It is based on the idea that electron pairs in a molecule will repel each other and will arrange themselves in a geometry that minimizes this repulsion.
This theory is used to explain the shapes of molecules and the angles between their bonds. It has many applications in chemistry, including predicting the behavior of molecules in chemical reactions, understanding the physical and chemical properties of molecules, and designing new molecules with desired properties.
The most common application of VSEPR theory is predicting the shapes of molecules. The theory states that the electron pairs in a molecule will arrange themselves in a geometry that minimizes their repulsion. This is usually a three-dimensional structure with a central atom surrounded by other atoms or lone pairs.
The number of electron pairs and their arrangement determines the shape of the molecule and the angles between the bonds. For example, the methane molecule (CH4) has four electron pairs and is, therefore, tetrahedral, with bond angles of 109.5°.
VSEPR theory is also used to explain the chemical properties of molecules. Since the electron arrangement affects the chemical properties of a molecule, VSEPR theory can be used to predict how molecules will react in a given environment.
For example, if a molecule has a bent geometry, it will likely react with other molecules at the ends of the bent shape. This can be used to explain the behavior of molecules in chemical reactions. Finally, VSEPR theory is also used in the design of new molecules with desired properties.
By understanding the shapes and angles of molecules, chemists can design molecules with specific properties. For example, molecules can be designed with specific bond angles to increase their reactivity or stability. This can be used to develop new and improved drugs, catalysts, and materials.
Overall, VSEPR theory is an important tool in chemistry that is used to predict the shapes and properties of molecules. It has many applications, including understanding the behavior of molecules in chemical reactions, predicting the physical and chemical properties of molecules, and designing new molecules with desired properties.
The Conclusion:
The VSEPR theory is a powerful tool for predicting the shapes of molecules and ions. It is based on the idea that electron pairs around a central atom will repel each other, causing the molecule to adopt a certain shape.
The theory is able to predict the shapes of simple molecules, such as methane and ammonia, as well as more complicated molecules, such as water and methanol.
The VSEPR theory is a useful tool for predicting the shapes of molecules and ions, but it has its limitations. It is not able to predict the exact geometry of molecules with more than four atoms or molecular shapes that are affected by lone pair electrons or hydrogen bonding. Furthermore, it does not take into account the effects of electrostatic forces or resonance structures.
Despite these limitations, the VSEPR theory is important to understanding chemistry. It is a valuable tool for predicting the shapes of molecules and ions and can be used as a starting point for more detailed investigations. Ultimately, the VSEPR theory provides a useful framework for understanding the structures of molecules and ions.
The Reference:
(Valence Shell Electron Pair Repulsion Theory) is a model used in chemistry to explain the geometry of molecules? It is based on the idea that the valence electron pairs around a central atom in a molecule will repel each other, causing the electron pairs to arrange themselves as far apart as possible.
The concept of VSEPR was first proposed by Sidgwick and Powell in 1940 and was further developed by Gillespie and Nyholm in 1957. This work was based on the assumption that electron pairs in a molecule would repel each other, causing them to move to the positions of maximum separation.
These positions can be determined by considering the angles between the electron pairs. The basis of VSEPR theory is the concept of electron-pair repulsion. This idea states that electron pairs, either lone pairs (non-bonding pairs) or bonding pairs (those involved in covalent bonds), will repel each other, resulting in a minimized energy state.
This energy minimization results in the electron pairs assuming positions of maximum separation. The angles between the electron pairs can then be used to determine the molecular geometry of the molecule. VSEPR theory has been used to explain the shapes of molecules ranging from simple diatomic molecules to complex polyatomic molecules.
It has been found to be very successful in predicting the structure of molecules and has been used to explain the properties of many compounds.
VSEPR theory has been further refined and developed over the years and continues to be used to explain the properties of molecules. It has been recognized as a powerful tool for predicting the structure of molecules and has been widely accepted by chemists.
References: Sidgwick, N.V. and Powell, G.H. (1940). “
The structure of small molecules and ions”.
Proceedings of the Royal Society A, 176, pp. 232–242.
Gillespie, R.D. and Nyholm, R.S. (1957).
“The structure of molecules determined by the valence shell electron pair repulsion (VSEPR) theory”.
Proceedings of the Royal Society A, 241, pp. 16–36.
Walsh, P.J. (2003). Molecular Structure and Properties.
Oxford University Press. Wells, A.F. (1984).
Structural Inorganic Chemistry. Oxford University Press.
Oxtoby, D.W., Gillis, H.P. and Nachtrieb, N.H. (2007).
Principles of Modern Chemistry. Cengage Learning.
Also, read Objectives of Auditing




























Comment on “VSEPR theory”
Comments are closed.