Table of Contents
There are mainly two types of charging methods in Automobiles namely constant voltage charging and constant current charging:
Constant Voltage Charging:
In this method, the charging voltage is kept constant throughout the charging process. In this method, the charging current is high at first, when the battery is discharged, and gradually drops off as the battery picks up the charge, resulting in an increase in return emf.
Charging at constant voltage can only be carried out when the batteries have the same voltage, for example, 6 or 12, or 24 V. In this case, the current source must have a voltage of 7.5, 15, or 30 V; these batteries are connected in parallel to the charging circuit.
The convenience of charging at constant voltage is that it allows cells with different capacities and different degrees of discharge to be charged. The large charging current at the start of charging is relatively short-lived and does not harm the cells.
At the end of charging, the charging current drops to almost zero because the battery voltage becomes almost equal to the supply circuit voltage. This method, however, is not very suitable for old, badly sulfated batteries that need prolonged charging at a slow rate.
This method is the most common method of charging lead-acid batteries and has been used successfully for over 50 years for different types of lead-acid batteries. With this charging method, charging time is almost halved, capacity is increased by approximately 20%, but efficiency is reduced by approximately 10%.
Constant current charging:
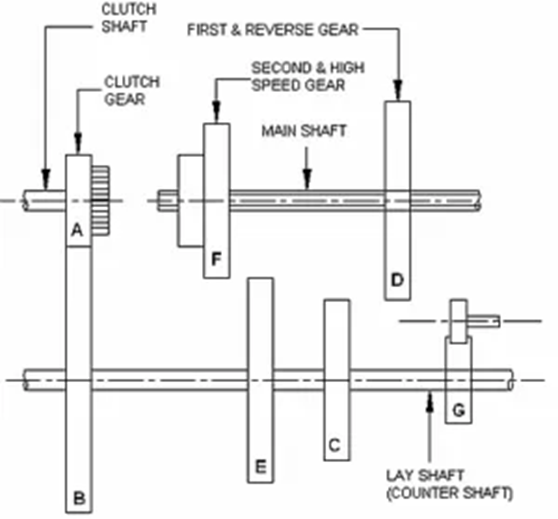
In this method of charging batteries, the batteries are connected in series to form groups and each group is charged from the DC supply network through charging rheostats. The number of batteries in each group depends on the voltage of the charging circuit, which must not be less than 2.7 V per cell.
The charging current is kept constant throughout the charging period, reducing the resistance in the circuit as the battery voltage increases. This method is generally used for the initial charging of lead-acid batteries and for charging portable batteries in general.
In order to avoid over-gassing or overheating, charging can also be performed in two steps, a comparatively higher current initial charge, and a low current finishing rate. In this method, the charge current is maintained at one-eighth of its ampere-hour rating.
Excess voltage from the supply circuit is absorbed in the series resistor. However, the battery packs to be charged must be connected in such a way that the series resistor absorbs as little energy as possible. The series resistance used must be of current carrying capacity equal to or greater than the required load current; otherwise, the series resistor will overheat and burn.
Battery groups must be selected so that they all have the same capacity. If the batteries have different capacities, the charging current should be set according to the battery with the smallest capacity. This would slow down and complicate the charge.
Charging Indications for Lead Acid Battery:
The total charge of the lead-acid accumulator (or cells) can be evaluated from the following indications:
1. Gassing:
When the cell is fully charged, hydrogen and oxygen gases are released at the cathode and anode respectively, so the release of gases (hydrogen and oxygen), known as gasification, at the electrodes indicates that the cells are fully charged.
2. Colour:
When the cell is fully charged, the lead sulfate anode is converted to lead peroxide (PbO2) in dark chocolate brown color, and the lead sulfate cathode is converted to lead (Pb), in grey color. It is considered one of the best tests to check the status of a battery.
3. Voltage:
When the cell is fully charged, its terminal potential will be approximately 2.6 volts.
Alkaline batteries:
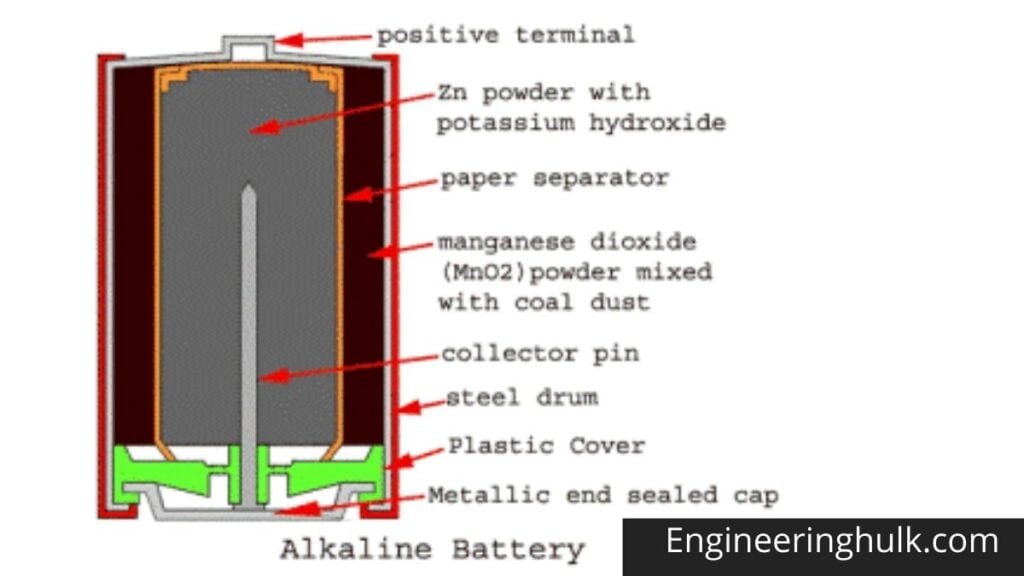
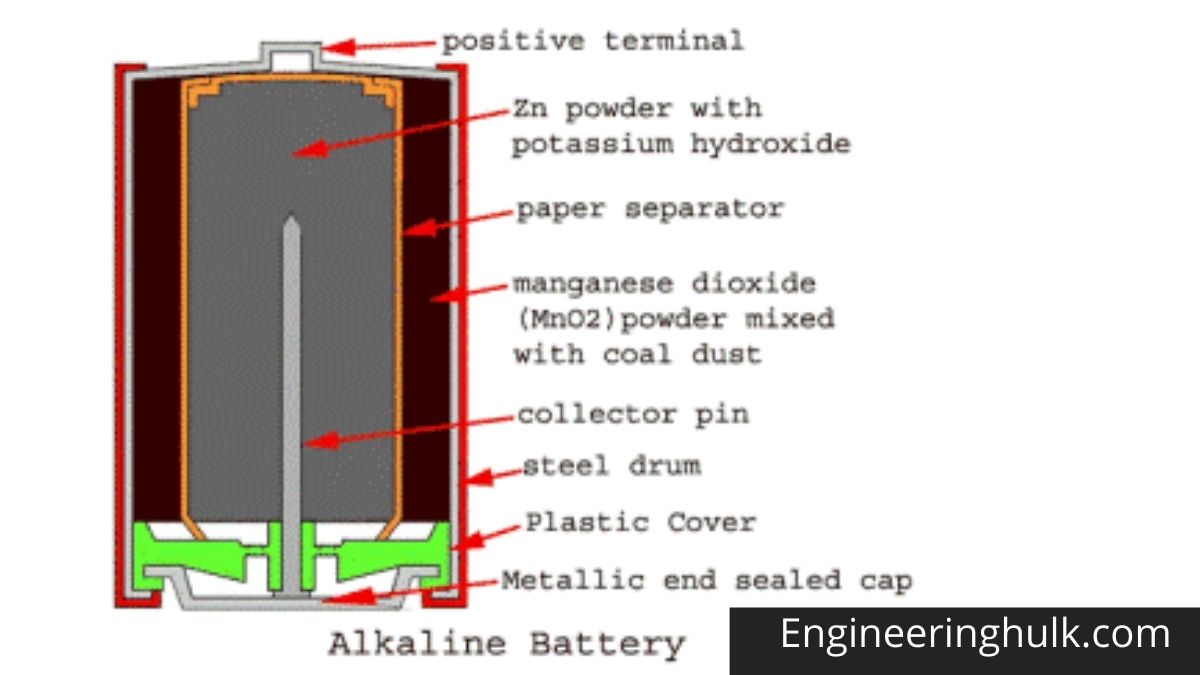
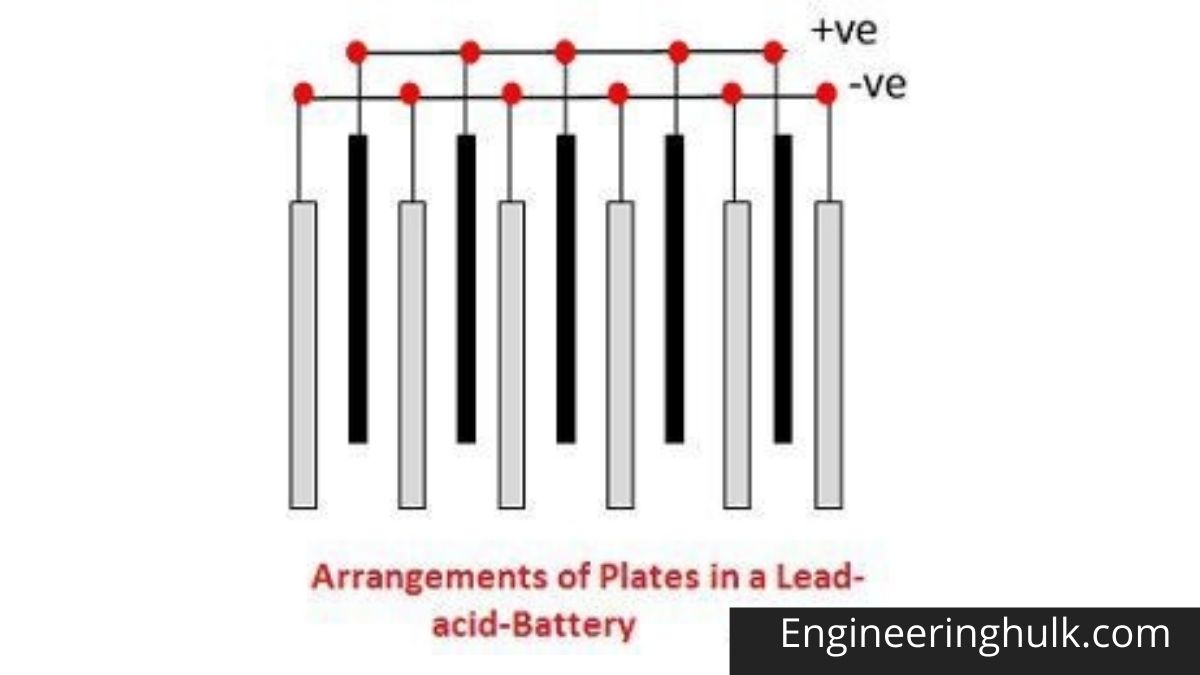
The drum body is made of a hollow steel drum. This drum contains all battery materials and also serves as the battery cathode. The positive battery terminal is projected from the top of this drum. Fine-grained manganese dioxide powder (MnO2) mixed with coal powder is transferred to the inner peripheral surface of the empty cylindrical drum. This molded mix serves as an alkaline battery cathodic mix.
The inner surface of the thick layer of the cathodic mixture is covered with a paper separator. The central space inside this paper separator is filled with zinc dust with potassium hydroxide electrolyte. Zinc serves as an anode and its powder form increases the contact surface. The paper separator soaked in potassium hydroxide keeps the electrolyte between the cathode (MnO2) and the anode (Zn).
A metal pin (preferably made of brass) is inserted along the central axis of the alkaline battery to collect the negative charge. This pin is called a negative collector pin. This pin is in contact with a sealed metal cap. There is a plastic cap just inside the sealed metal cap, and this plastic cap electrically separates the positive steel drum and the negative alkaline battery cap.
There are several types of alkaline batteries, depending on various parameters.
Depending on the composition of the active materials of the plates, there are four types of batteries. They are as follows,
- Nickel iron (or Edison).
- Nickel-cadmium (or Nife).
- Silver zinc.
- Alkum battery
Used in commercial airlines, and military aircraft to start the main engine. Thus, we can say that the alkaline battery is mainly used in moving vehicles and for industrial purposes.
Advantages of Alkaline Batteries
- This has a high energy density.
- This battery works equally well in continuous and intermittent applications.
- This works equally well at low and high discharge rates.
- This also works just as well at room temperature as at low temperatures.
- Alkaline battery also has low internal resistance.
- It has a life of its own long enough.
- Leakage is low on this battery.
- It has better dimensional stability.
- This type of battery has no disadvantages other than its high cost.





















Comment on “Battery Charging – Methods, Advantages, & Disadvantages”